Research Overview
1. Drug Discovery Based on Original Molecules
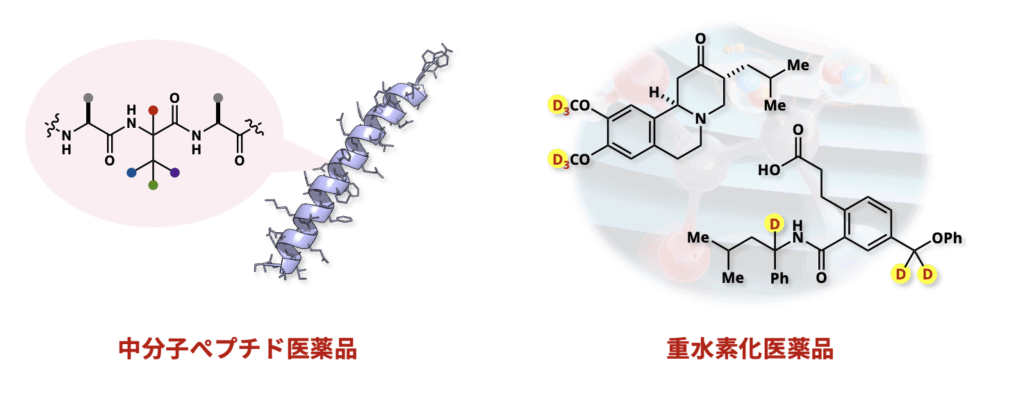
Protein–protein interactions (PPIs) play central roles in regulating a wide range of biological processes both inside and outside the cell, and are therefore considered highly attractive targets in drug discovery. However, due to their large and flat binding surfaces, PPIs are often difficult to inhibit using conventional small-molecule drugs, and the development of effective inhibitors has long been a challenge.
To address this issue, medium-sized peptides have recently attracted attention as a promising new modality. Owing to their relatively large and flexible structures, peptides can bind with high affinity to the broad interfaces characteristic of PPIs, making them well-suited for this purpose. In our group, we are pursuing drug discovery research based on original molecules and synthetic methodologies that we have uniquely developed. In particular, we focus on the design and synthesis of medium-sized peptides containing unnatural amino acids, aiming to discover potent peptide-based PPI inhibitors.
Through close collaboration with researchers from various fields, we are working on the development of highly bioactive, original molecules and advancing their applications in drug development.
We are also developing new synthetic methods for the preparation of deuterated drugs, in which specific hydrogen atoms are replaced with deuterium (D). Deuterium has physicochemical properties similar to hydrogen but can slow metabolic degradation, leading to improved drug stability, reduced dosage, and potentially fewer side effects. We aim to establish new methods that enable site-selective and precise deuterium incorporation, previously considered technically challenging, and apply them toward the practical development of next-generation deuterated pharmaceuticals.
In this way, we promote drug discovery through a bottom-up approach based on organic synthesis, striving to create innovative medicines from precisely designed and synthesized molecules.
2. Novel Transformations Using Radicals
Radical reactions are characterized by their generally fast kinetics and tolerance to steric hindrance, making them particularly attractive for constructing complex molecular architectures that are often difficult to access via ionic (anionic or cationic) pathways.
However, when radical reactions are conducted in a catalytic and controlled manner, competing side reactions such as homocoupling or undesired chain processes often occur, making it difficult to achieve high selectivity for the desired product.
Our group is working on the catalytic control of carbon-centered and nitrogen-centered radicals to develop useful molecular transformation reactions. By harnessing the unique reactivity of radicals under controlled conditions, we aim to contribute to the synthesis of structurally and functionally intriguing organic molecules.


